The Prognostic Value of Nailfold Capilla
Teks penuh
Gambar
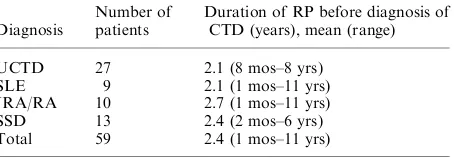
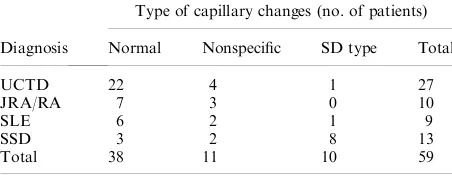
Dokumen terkait
Memberikan gambaran kepada orang tua khususnya ibu, tentang hubungan persepsi dengan keterlibatan yang mereka miliki serta lebih memperhatikan pengembangan literasi
Berdasarkan hasil bahwa tidak terdapat hubungan antara kejadian malaria dengan kadar feritin(lihat tabel 5.6).Hasil penelitian ini sama dengan hasil Penelitian yang
Selain koefisien determinasi juga didapat koefisien korelasi yang menunjukkan besarnya hubungan antara variabel bebas yaitu pemahaman peraturan pajak, tarif pajak, lingkungan,
Satu set tempat tidur king-sized (lengkap dengan base-nya), satu mesin cuci kapasitas 6 kg dan satu kulkas dua pintu yang masih bagus bisa diperoleh dengan harga AUD 600
menggunakan pendekatan penelitian kualitatif untuk memahami fenomena tentang apa yang dialami oleh subjek penelitian, seperti subjek, persepsi, motivasi, tindakan,
Untuk mengatasi kesulitan tersebut Villardon (Jordana & Sànchez, 2010) menawarkan untuk menggunakan berbagai rangkaian aktivitas evaluasi untuk mengases kegiatan
sungai dengan suhu ini dapat hidup di permukaan, tengah maupun dasar perairan dengan suhu 27-28 o C dengan pH 6. yang hidup di Sungai Parit Belanda merupakan