ijph suppl 1 2017 web approval
Teks penuh
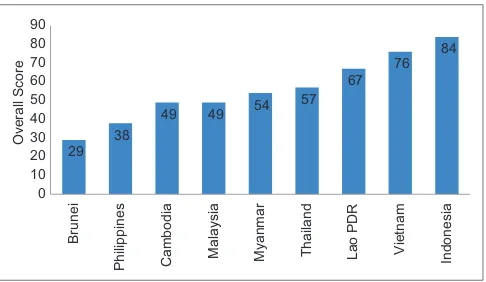
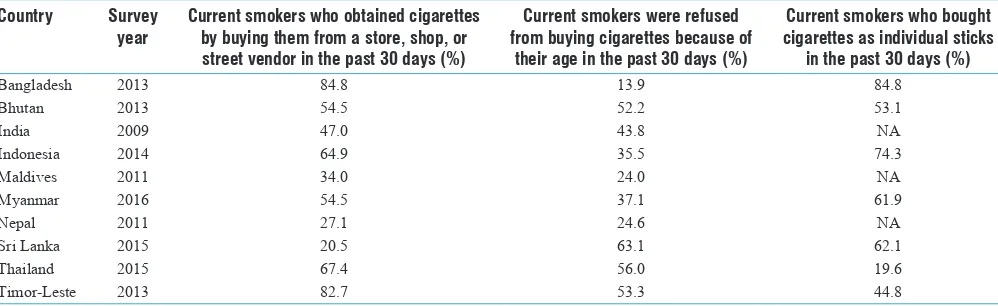
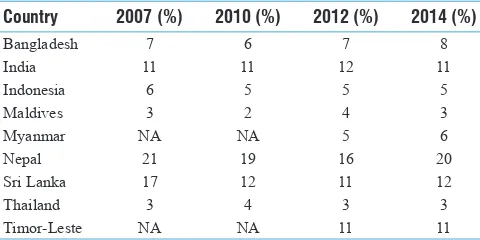
Gambar


Garis besar
Dokumen terkait
Harianto Farried dan Sudomi Siswanto, Perangkat dan Teknis Analisis Investasi di Pasar Modal Indonesia (dalam Skripsi : Juli Gemili, Perlindungan Hukum Terhadap Investor Atau
[r]
Adapun aspek kecerdasan emosi menurut (Goleman, 2000) yaitu: a) Mengenali Emosi Diri merupakan suatu kemampuan untuk me- ngenali perasaan sewaktu perasaan itu
Penelitian ini sangat penting dilakukan karena perilaku prososial remaja khususnya yang terjadi di SMP Cita Hati Surabaya perlu diteliti untuk mendapatkan
Pada penelitian dan pembuatan alat ini ditujukan untuk mengetahui seberapa kuatkah infrared dalam memancarkan sinarnya sebagai titik awal dalam penentuan waktu
Pada hari ini, Kamis tanggal Enam belas Bulan Maret Tahun Dua ribu tujuh belas (16-03- 2017) Panitia Pengadaan Jasa Pemborongan Pekerjaan Peningkatan Kapasitas Gerbang Tol Sidoarjo
Arkoun mengemukakan anggapan yang mengandung tiga unsur penting: pertama , ia menghubungkan proses pembekuan dan penutupan dalam penafsiran Alquran dengan pengalihannya dari
• Undangan Presentasi hanya disampaikan kepada Peserta Penawaran yang LULUS tahapan Evaluasi Administrasi. • Apabila Peserta Tidak upload Dokumen penawaran atau Terlambat