MAKALAH SEMINAR NASIONAL SOEBARDJO BROTO
Teks penuh
Gambar
Dokumen terkait
Pada hari ini Jumat Pikul 08.50 tanggal Dua Puluh Enam tahun Dua Ribu Tujuh Belas , kami Kelompok Kerja Pengadaan Barang/ Jasa lainnya Bagian Layanan Pengadaan
Output amplifier sebesar 30 dBm akan dilewatkan kabel dengan redaman / loss 2 dB.. A power amplifier with a 40 dB gain has an output power of
Berdasarkan Ketentuan Perpres nomor : 54 tahun 2010 dan Perpres nomor : 70 tahun 2012 beserta perubahannya dan Dokumen Pengadaan nomor : 027.480/SBD/POKJA- ULP/IX/2014 tanggal :
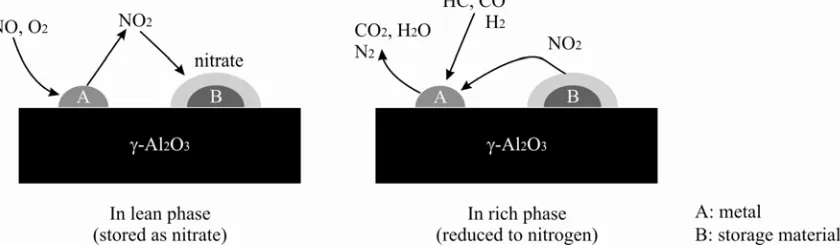
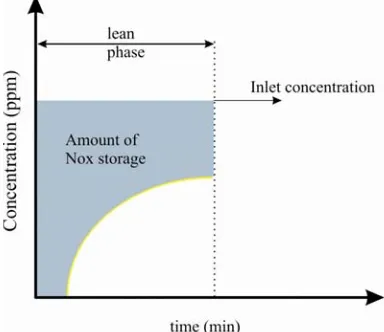
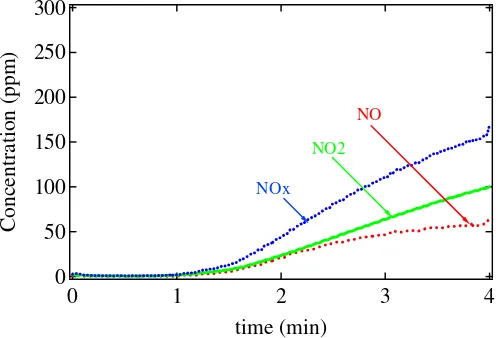
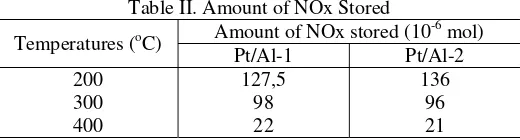
[r]
[r]
Dengan menggunakan teks yang telah ditulisi bimbingan guru, siswa dapat membacakan cerita narasi yang telah ditulis tentang persiapan berwisata ke pantai dengan lafal dan
Seluruh asli dokumen penawaran Saudara yang telah diunggah melalui LPSE Kota Medan;.. Berkas asli Dokumen Kualifikasi dan fotokopinya sebanyak 1
Overall, the widening foreign- native gap in mean wages with the number of years spent in the United States in recent years is mostly driven by Latin American and Asian immigrants