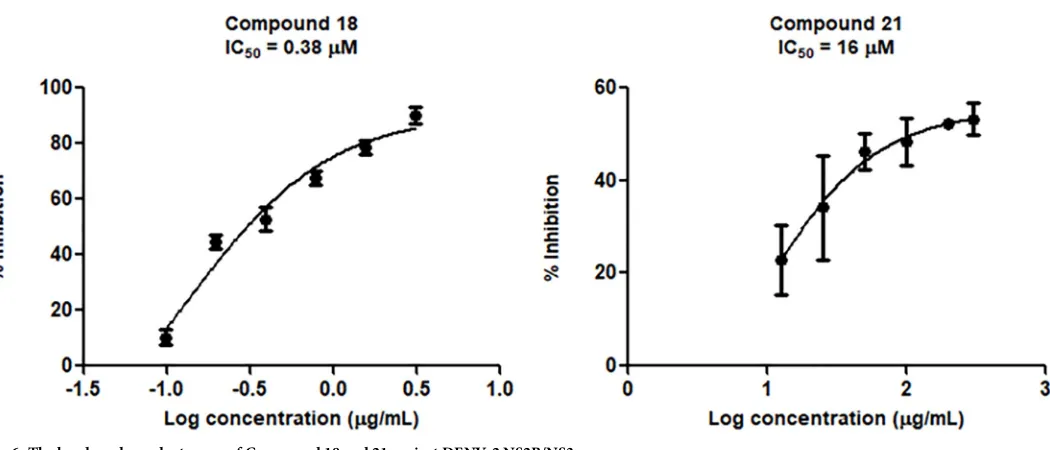
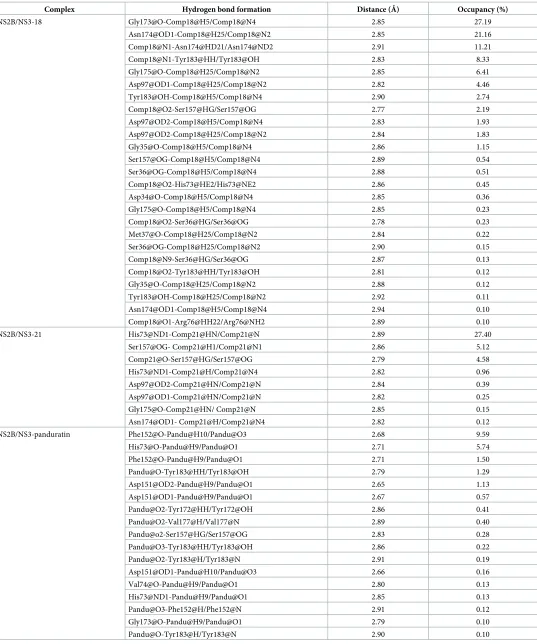
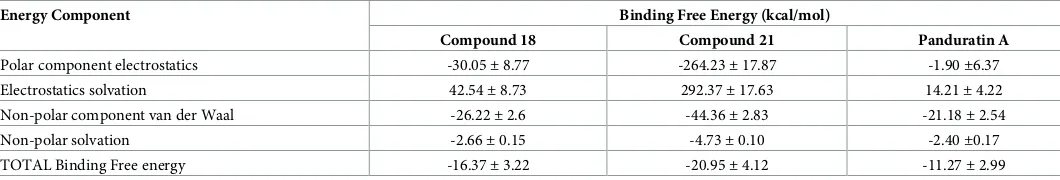
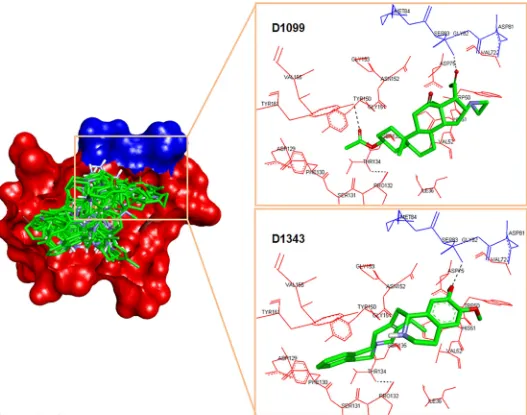
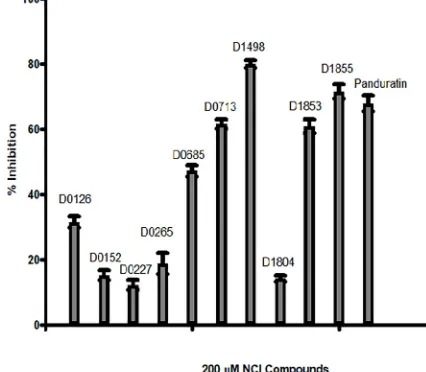
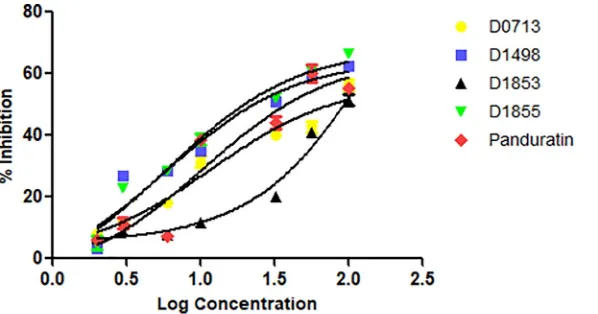
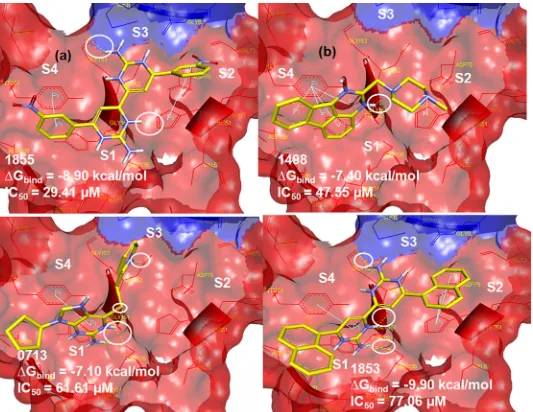
Thioguanine-based DENV-2 NS2BNS3 protease inhibitors: Virtual screening, synthesis, biological evaluation and molecular modelling
Teks penuh
Gambar
Dokumen terkait
Dalam wawancara n pen€lrtltidal mehbe.kan penlarahan'penoaahan yanq tajam, akan tetapi terserah kepada nlorman yanq dw8wa.@ra untuk memberkan oenFa$n menlrlt
11.. kemampuan empati lebih mampu menangkap sinyal-sinyal sosial yang tersembunyi yang mengisyaratkan apa-apa yang dibutuhkan orang lain sehingga ia lebih mampu menerima sudut
Bab ini berisi hasil implementasi dari hasil analisis dan perancangan sistem yang telah dibuat disertai juga hasil pengujian sistem yang dilakukan di Dinas
Tujuan penelitian untuk menilai efek ekstrak etanol buah alpukat (EEBA) dalam menurunkan kadar kolesterol LDL tikus wistar dan membandingkan potensi ekstrak etanol
DOSIS INOKULUM DAN T.AMA TERMtrNTASI AMfAS SACII.. TIiRIIADAP BATTA.N
[r]
13 Di Yogyakarta, HIS Katolik mulai didirikan tahun 1918 oleh Pastor van Driessche, SJ., yang bertujuan selain menampung anak-anak Jawa, juga sebagai tempat latihan bagi
Nilai Total HPS : Rp 199.266.000,00 ( Seratus Sembilan Puluh Sembilan Juta Dua Ratus Enam Puluh Enam Ribu Rupiah ).