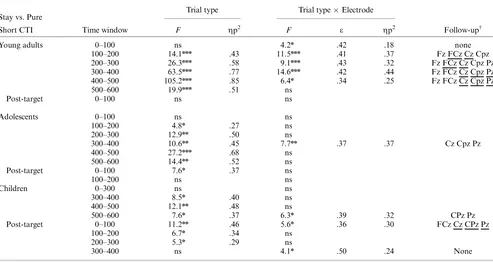
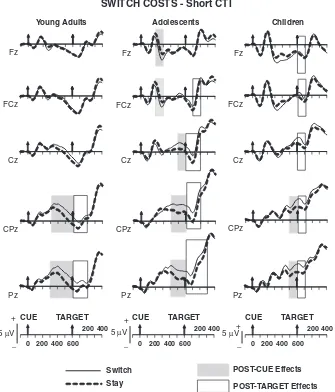
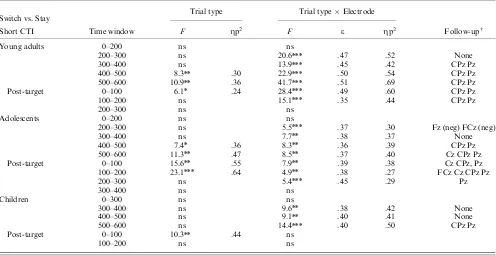
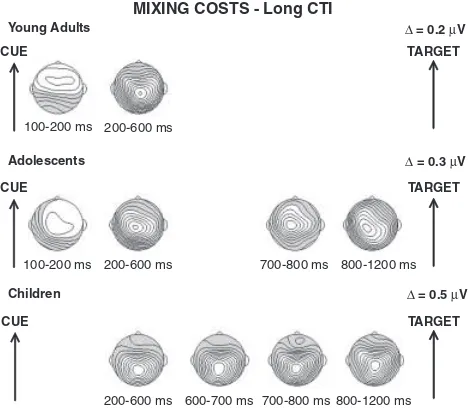
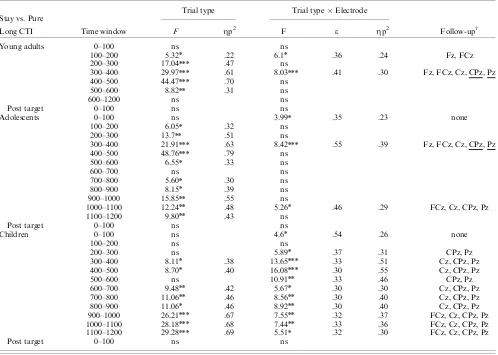
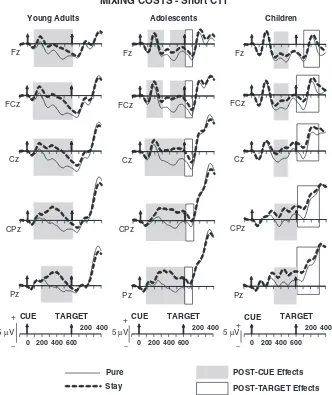
The development of anticipatory cognitiv
Teks penuh
Gambar
Garis besar
Dokumen terkait
Kepada peserta lelang yang keberatan dengan pengumuman ini diberikan kesempatan untuk menyampaikan sanggahan melalui aplikasi SPSE kepada Pokja I Unit Layanan Pengadaan
Berdasarkan hasil penilaian yang telah dilakukan oleh Kelompok Kerja Direktorat Budidaya Ternak terhadap Dokumen Penawaran File I meliputi meliputi Dokumen
It is thus not possible to construct the com- ponent of the vacant set containing the root of a given tree as a sequence of independent genera- tions, which is a key property
Ada empat kategori yang jadi target pengangkatan PNS yaitu pengangkatan te naga honorer, pegawai tidak tetap, pegawai tetap non PNS, dan tenaga kontrak menjadi PNS.. Menurut Ketum
Buku/Dokumen Manual SPMI adalah dokumen berisi petunjuk teknis tentang cara, langkah, atau prosedur Penetapan, Pelaksanaan, Evaluasi pelaksanaan,
Demikian Berita Acara Penyerahan Barang/jasa ini dibuat dengan sebenarnya dalam rangkap 3 (tiga) untuk dipergunakan seperlunya.. Pihak Kedua Direktur PT/CV……… Pihak Pertama
Di bawah ini imunisasi yang bisa diberikan pada pasien yang..
Semua anggota kelompok kembali ke kelompok yang semula dan melaporkan apa yang mereka temukan dari kelompok lain.. setiap kelompok kemudian membandingkan dan membahas