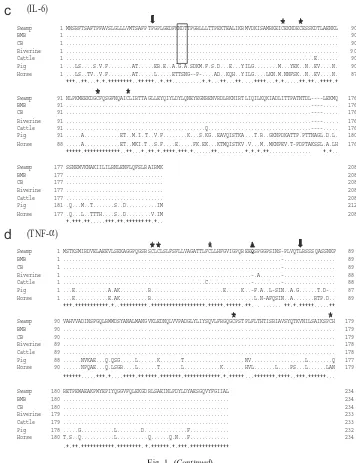
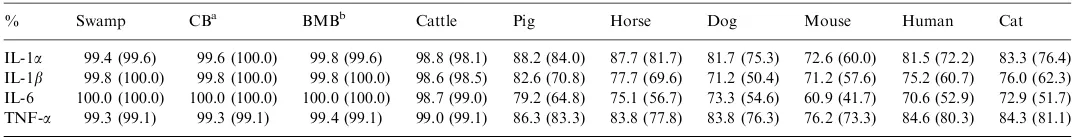
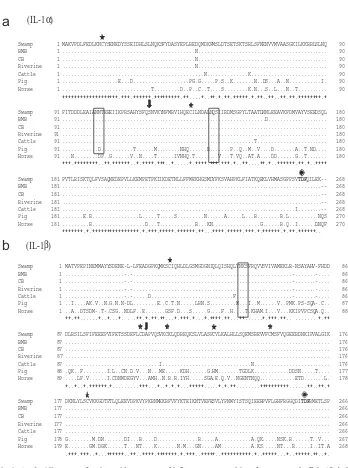
molecular cloning sequencing and phylogenetic
Teks penuh
Gambar


Garis besar
Dokumen terkait
Hasil penelitian menunjukkan bahwa : (1) Perbedaan laba fiskal berpengaruh negatif terhadap persistensi laba sedangkan arus kas operasi dan kepemilikan
PEMERINTAH KOTA TANGERANG SATUAN KERJA PERANGKAT DAERAH.. Urusan Pemerintahan
dalam apel tersebut Kapolres Takalar mengucapkan terimaksih kepada Seluruh Personel Polres Takalar baik terlibat langsung mengikuti Trail Adventure 2017 Jelajah Butta
[r]
TMS.TL.TA.2 = Gugur Evaluasi Admintrasi kerana ; Tidak Mengupload/ Scan Surat Dukungan Penyediaan Geotextille dari Distributor/Agen yang disertai Brosur. PDF created
Berdasarkan nilai indeks geoakumulasi di Teluk Buli untuk logam berat Pb, Cd, Cu, Ni, Zn dan Fe nilai rerata seluruh logam berat lebih kecil dari 0 (I_geo<0),
Indonesia Nomor 3 Tahun 2003 Peranan Teknologi Informasi dan Komunikasi (TIK) dalam penyelenggaraan pemerintahan, maka Pemerintah Republik Indonesia telah menyusun
Berdasarkan karakteristik responden dengan dimensi tipe kepribadian menunjukkan tidak terdapatnya hubungan antara tipe kepribadian introvert dan ektrovert dengan